EU Approves Sales Of Artificial Heart
January 17, 2021
Artificial heart maker Carmat will begin sales of its devices from the second quarter of this year after a long-awaited European Commission approval.
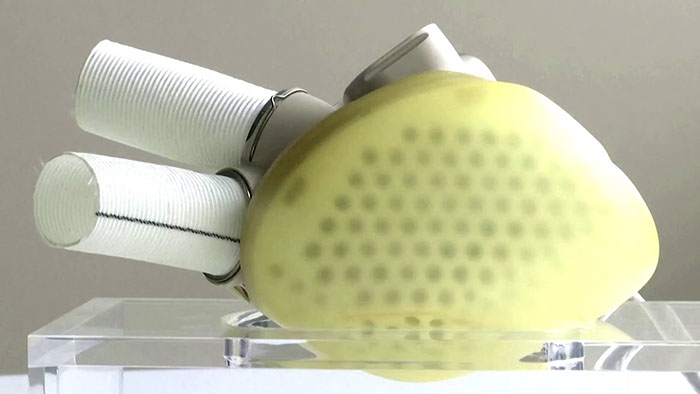
The device aims to give patients with end-stage biventricular heart failure, a deadly condition where the heart is no longer able to pump blood adequately around the body, an alternative to hospital stays.
Carmat calls its device "the world's most advanced total artificial heart project" although they are not the first company to create artificial hearts. US group Syncardia has been selling an artificial heart for nearly two decades.
"Syncardia is very important in the history of artificial hearts because they proved that it's possible, and it works, to change a human heart for a device," Carmat's chief executive, Stéphane Piat, says. "But Syncardia's is a very old technology and we are very far from what they are doing."
"If you compare our device to what has been achieved so far with other solutions, we are much better and we have no complications," he told Vantage.
Carmat's device, to be marketed under the brand name Aeson, will be made available in Germany, possibly followed by France and other countries.
It is also launching a study in the United States this quarter with the hope of getting approval from the Food and Drug Administration for its device by 2024.
Watch the video below.
 Dogs Are Forced To Wear The Things They Steal — And It’s Hilarious
Dogs Are Forced To Wear The Things They Steal — And It’s Hilarious
 She Was Feeling Low On Confidence, So Her Classmates Covered Her Desk In Sticky Notes
She Was Feeling Low On Confidence, So Her Classmates Covered Her Desk In Sticky Notes
 This Baby Giraffe Named Eugene Was Born With The Most Hilarious Tuft Of Hair You’ve Ever Seen
This Baby Giraffe Named Eugene Was Born With The Most Hilarious Tuft Of Hair You’ve Ever Seen
 It Looks Like Her 2-Year-Old Ruined Her Doll — But Then Mom Shows Why It’s Perfect
It Looks Like Her 2-Year-Old Ruined Her Doll — But Then Mom Shows Why It’s Perfect
 On February 28, A Rare Planetary Parade Will Appear In The Evening Sky
On February 28, A Rare Planetary Parade Will Appear In The Evening Sky
 Man Discovers Rare Mammoth Bone In Missouri
Man Discovers Rare Mammoth Bone In Missouri
 Reporter Asks Eileen Gu 'Do You Think Before You Speak?' — Her Answer Is Going Viral
Reporter Asks Eileen Gu 'Do You Think Before You Speak?' — Her Answer Is Going Viral
 A Mom Saw A Soldier Who Didn't Have Any Family... So She Hugged Him
A Mom Saw A Soldier Who Didn't Have Any Family... So She Hugged Him
 Man Helps Distressed Swan Find Her Way Back To The River
Man Helps Distressed Swan Find Her Way Back To The River
 This Father-Daughter Game Ends With Everyone In Tears
This Father-Daughter Game Ends With Everyone In Tears
 'These Are From Dad': Before He Died, He Asked His Daughter To Do This For Her Mom Every Year
'These Are From Dad': Before He Died, He Asked His Daughter To Do This For Her Mom Every Year
